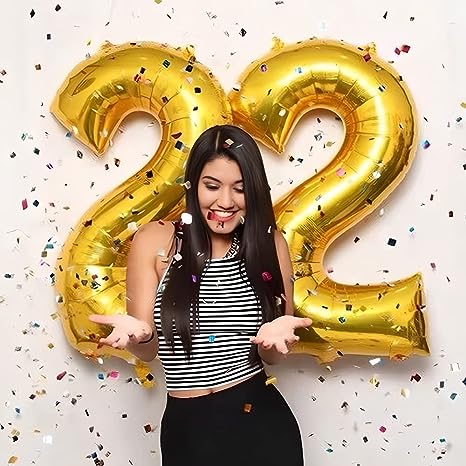Feedback
Segnala
Insight
Celestia Il Richiamo del Cosmo - In un angolo nascosto dell'universo, un pianeta di nome Celestia brilla come una stella luminosa. Ma c'è una donna straordinaria: Astrid. Formato Kindle
 Cuore e Tavola - Penso dunque sono! Green: Guida Verso una Vita più Verde tra Vegetali Ambiente e Stili di Vita con Ricette e Consigli
Cuore e Tavola - Penso dunque sono! Green: Guida Verso una Vita più Verde tra Vegetali Ambiente e Stili di Vita con Ricette e Consigli
 Tazza con Miscelatore/Auto Magnetic
Tazza con Miscelatore/Auto Magnetic
 Nespresso Inissia EN80.B, Macchina da caffè di De'Longhi, Sistema Capsule Nespresso, Serbatoio acqua 0.7L, Nero
Nespresso Inissia EN80.B, Macchina da caffè di De'Longhi, Sistema Capsule Nespresso, Serbatoio acqua 0.7L, Nero
 Ponmoo Foil Palloncini Numeri 2 Oro, Gigante Numero 0 1 2 3 4 5 6 7 8 9 10-19 20-29 30 40 50 60 70 80 90 100, Gonfiabili Pallone per Anniversario, Decorazione Feste di Compleanno Palloncino
Ponmoo Foil Palloncini Numeri 2 Oro, Gigante Numero 0 1 2 3 4 5 6 7 8 9 10-19 20-29 30 40 50 60 70 80 90 100, Gonfiabili Pallone per Anniversario, Decorazione Feste di Compleanno Palloncino
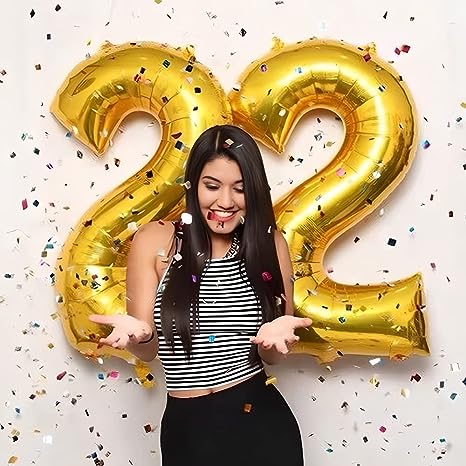
 Kingston Canvas Select Plus
Giochi e accessori per PC
Kingston Canvas Select Plus
Giochi e accessori per PC
 Orologi donna
Orologi donna
 Cosa devo fare per Creare, Produrre Criptovalute di successo: Guida alla creazione: Monete digitali, Regolamentazione, legalità e Glossario Criptovalute
Cosa devo fare per Creare, Produrre Criptovalute di successo: Guida alla creazione: Monete digitali, Regolamentazione, legalità e Glossario Criptovalute
 Diario dei Talenti - Poesie per l'anima: pensieri quantici, aforismi e poesie per l’anima
Diario dei Talenti - Poesie per l'anima: pensieri quantici, aforismi e poesie per l’anima